Informed Consent Accountability
Fully informed consent is not being obtained by most testing/injecting sites.
Failure to provide full information about of risks and benefits of a medical procedure is a violation of federal and most state laws as well as the Nuremberg Code.
At best, some sites send an email to the candidate with a link to “FACT SHEET FOR RECIPIENTS AND CAREGIVERS,” a seven-page document that is unlikely to be read by most people.
However, if readers make it to page 3, the section on risks begins with the inaccurate statement, “There is a remote chance that the Pfizer-BioNTech COVID-19 Vaccine could cause a severe allergic reaction…. (emphasis added).
This risk is far from remote and many mobile units have been found to be inadequately prepared to treat anaphylactic shock.
Below, this warning, readers find a partial list of the minor side effects: fever, nausea, pain, and swelling. There is no mention of the risk of death, hospitalization, myocarditis, blood clots, abnormal menstrual bleeding and spontaneous abortion—all of which are being reported to VAERS in alarming numbers.
Oddly, the 30-page “FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS,” does not require that the injection administrators inform their clients of the more serious adverse reactions.
Instead, page 9 states only that the providers must report serious adverse reactions to VAERS, along with instructions.
It defines the serious adverse events that requiring reporting as:
- Death
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
While the adverse effects are listed in the instructions to providers, there is no requirement to inform the recipients of any but the most minor adverse events.
Below are links to two different questionnaires that help people understand informed consent, summarized by these 1o facts:
- All Covid-19 injections are experimental and none are approved, only “authorized” for emergency use.
- The injections are not technically “vaccines”
- The spike protein produced in the body by the injection is toxic
- The injections are unlikely to confer lasting immunity.
- Traditional vaccines require 12–20 years of safety testing
- No long-term safety studies exist.
- Children are given an adult dose
- The manufacturers and providers have no financial liability for injuries
- For under 30’s, the injection’s risk of death or adverse effects is greater than those of Covid-19
- Vaccine adverse reactions must be reported to the CDC.
Individuals who are considering taking the C-19 injection can evaluate their awareness of the information needed for truly informed consent here:
Those who have already received the C-19 injection can evaluate how well they were informed here:
A PDF of the 10 questions used in both quizzes is available to participants.
Additional Factors
Relative Risk
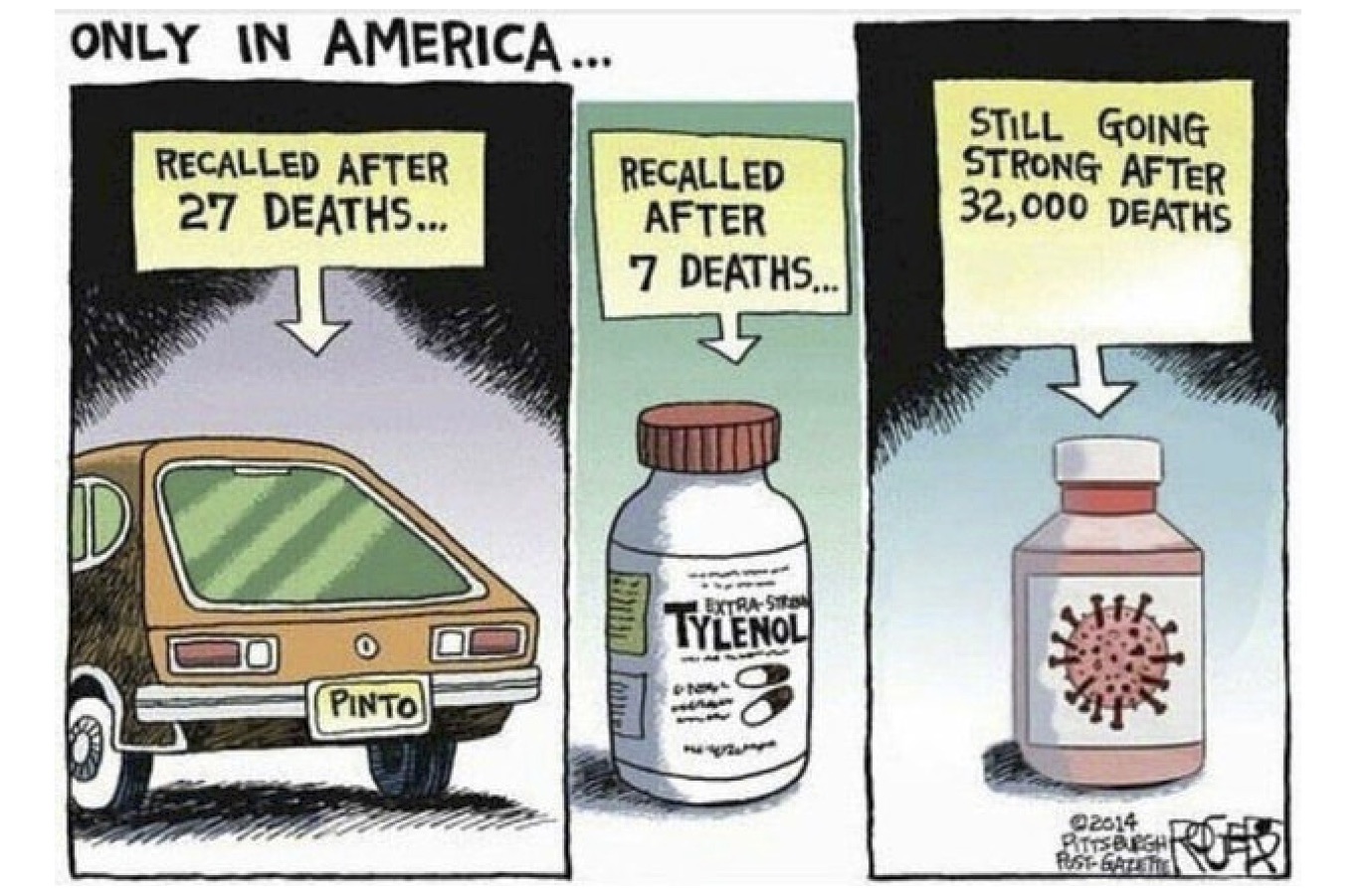
When the injections were first announced, their effectiveness was advertised as being 90-95%. What was shown was relative risk:

Comparing the red on the left to the red on the right shows the ratios of only the small number of those who actually were infected. This produces the 95% figure.
However, the pharmaceutical companies ignored the absolute risk findings from their studies.
Absolute Risk
When the numbers of infected people are compared to the entire population of 21,724, a very different picture emerges:
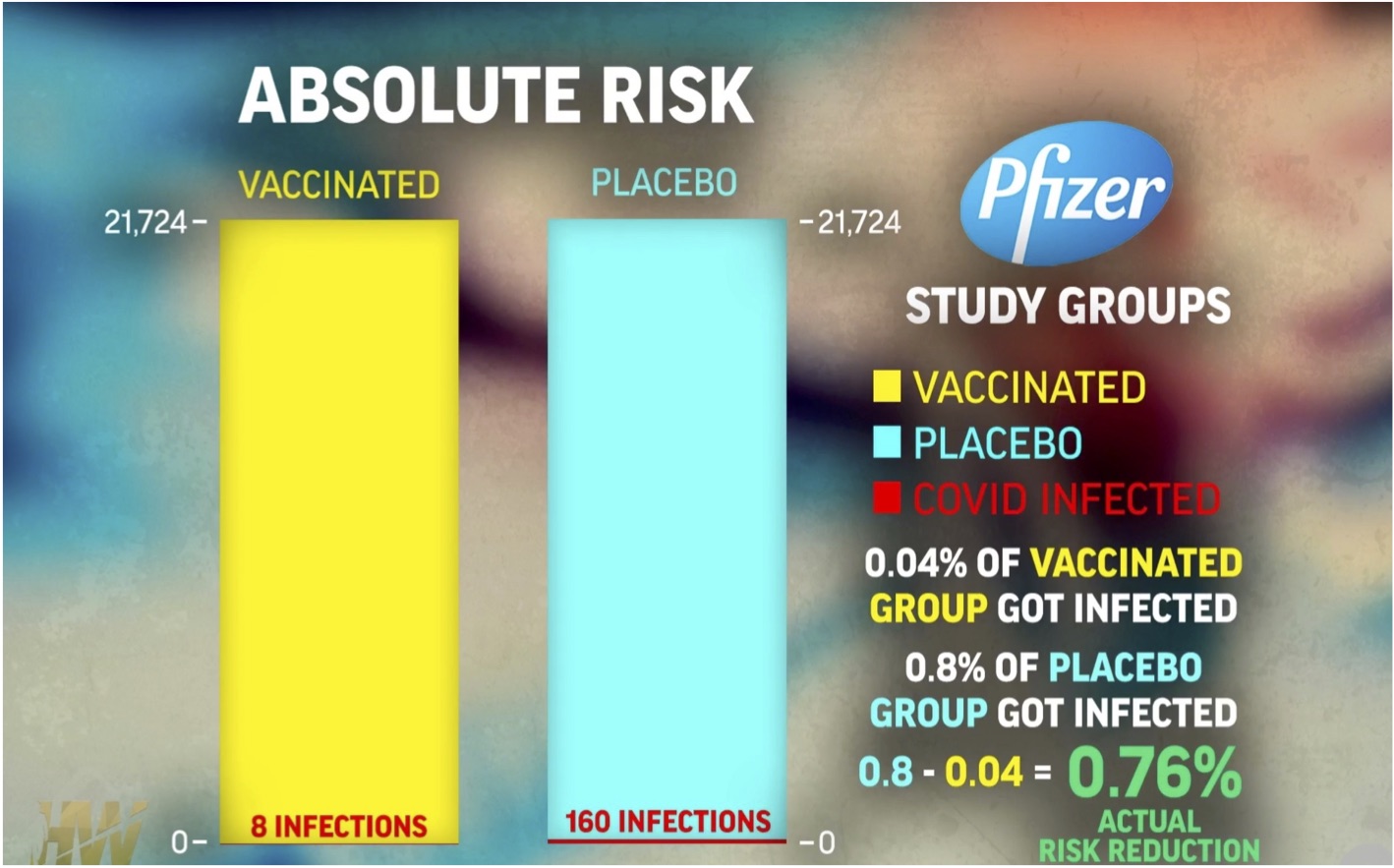
There is a dramatic difference.
Informed Consent—Three Metrics
True informed consent requires these three metrics:
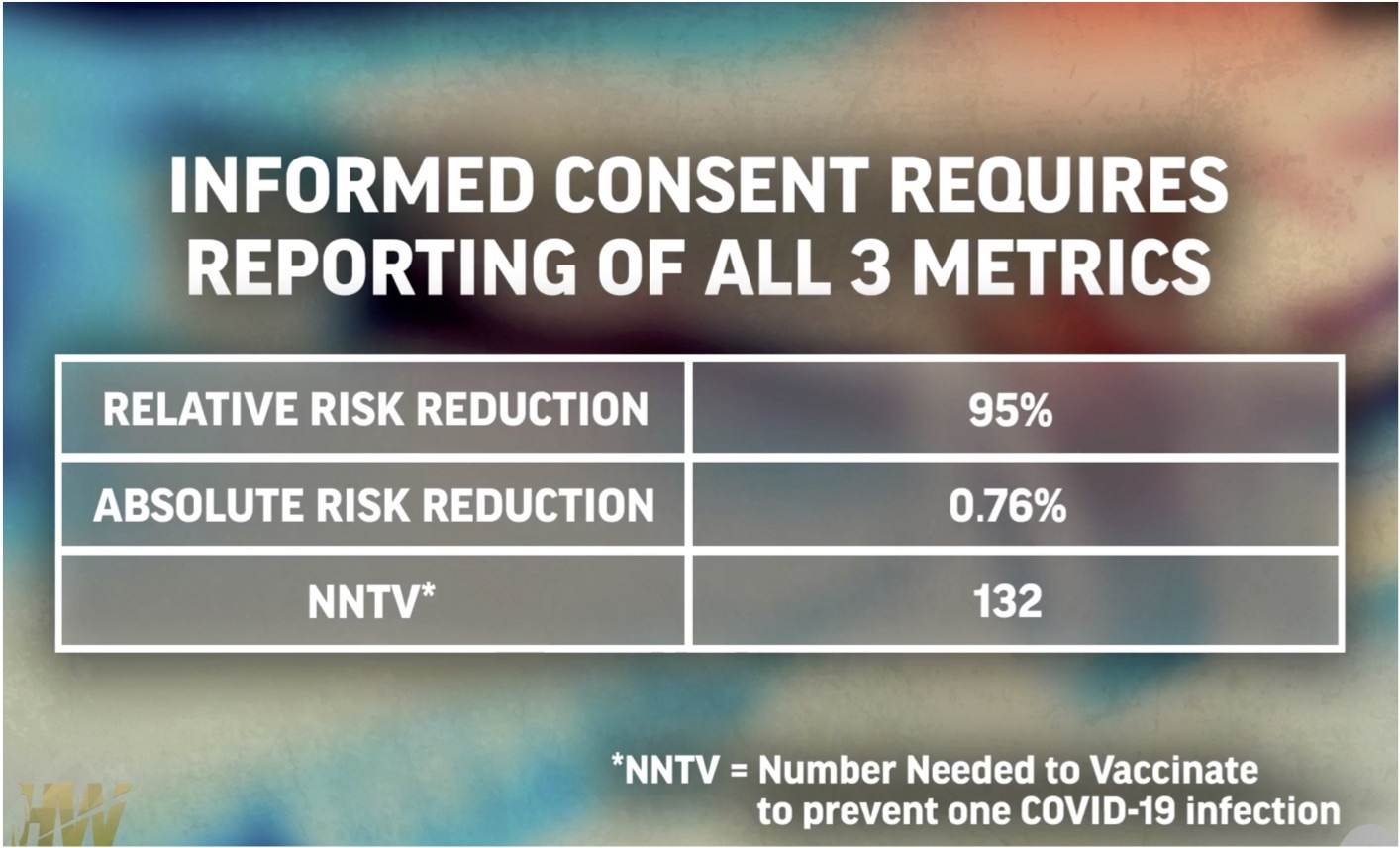
The third factor, NNTV means that 131 people must undergo the risks of the shot in order to protect one other person.
Conclusion